Kutshanje, iJiaxing Market Supervision Administration iqhube isampulu ebanzi yenkqubo yamanzi kaHaiyan Kangyuan Medical Instrument Co., LTD., Yaza yabhengeza ukuba inkqubo yamanzi yeKangyuan Medical ihambelana ngokupheleleyo neemfuno zamanzi asulungekileyo ze-2020 edition ye-Chinese Pharmacopoeia, eqinisekisa amandla okulawula okugqwesileyo kwimveliso yeKangyuan yokhuseleko lwezonyango.
Uhlolo lwesampulu lwaququzelelwa yiJiaxing Market Supervision Administration kwaye yagunyaziswa yiJiaxing Food, iChiza kunye noHlolo loMgangatho weMveliso kunye neZiko loVavanyo. Ngokuhambelana nemigangatho yesizwe echaphazelekayo kunye nemimiselo yoshishino, iZiko lokuhlola kunye novavanyo lwenze uvavanyo olubanzi kunye noluchwephesha kwinkqubo yamanzi esetyenziswa yiKangyuan Medical ukuvelisa izixhobo zonyango ezahlukeneyo, kubandakanywa amanzi pH, i-nitrate, i-conductivity, isinyithi esinzima, imida ye-microbial kunye nezinye iinkalo ezininzi. Emva kwemijikelo emininzi yovavanyo oluqatha, iziphumo zibonisa ukuba inkqubo yamanzi yeKangyuan Medical ihlangabezana ngokupheleleyo neemfuno zamanzi asulungekileyo zohlelo luka-2020 lwe-Chinese Pharmacopoeia, oluqinisekisa ngokupheleleyo ukhuseleko olusemgangathweni kunye nokuthembeka kweemveliso zesixhobo sonyango saseKangyuan.
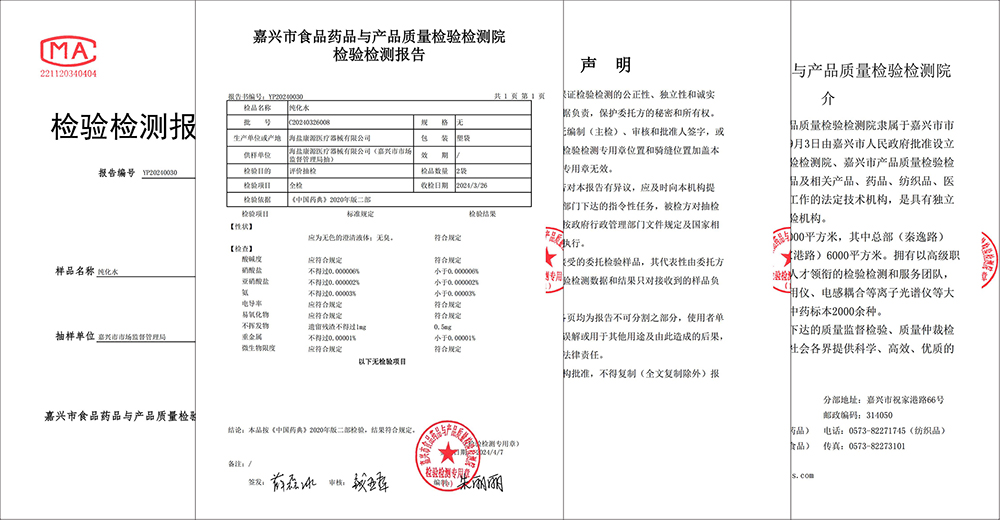
I-Kangyuan yezoNyango isoloko ibeka umgangatho wemveliso kunye nokhuseleko lwesigulane kwindawo yokuqala, kwaye igxininisa ukubaluleka kokulawulwa komgangatho wenkqubo yamanzi. Le nkampani iye yazisa izixhobo zokuvelisa amanzi ezikumgangatho ophezulu kunye nobuchwepheshe bokubeka iliso, kwaye yaseka inkqubo evakalayo yolawulo lwamanzi kunye neenkqubo zokusebenza zokuqinisekisa ukuba ithontsi ngalinye lamanzi liyahlangabezana nemigangatho yelizwe. Ukugqithiswa kokuhlolwa kwesampulu akukona nje ukuqinisekiswa komgangatho wamanzi enkqubo yonyango yaseKangyuan, kodwa kunye nokuqatshelwa kwenkqubo yokulawula umgangatho weKangyuan Medical.
Kwixesha elizayo, i-Kangyuan Medical iya kubamba ukuqokelela kwayo okunzulu kweshishini kunye nomoya wokuvuselela okuqhubekayo, ukuqhubeka nokudlala indima ephambili kwishishini lezonyango, ukuqinisekisa ukuba uninzi lwezigulane ezinomgangatho ongcono kunye nezinto ezisetyenziswayo zonyango ezikhuselekileyo, ukwenza igalelo elikhulu kwisizathu sempilo yabantu.
Ixesha lokuposa: May-29-2024

 中文
中文